Journal of Medicine, Engineering, Environmental and Physical Sciences (JOMEEPS), Vol. 1, No. 1, June-July 2023. https://klamidas.com/jomeeps-v1n1-2023-01/ |
||||||||
|
Protective Roles of Quercetin, Vitamin C and Pyridoxine on Lead Neurotoxicity via Enhanced Haematopoietic and Antioxidants Components Nnachi Ifenna Salvator, Elizabeth Finbarrs-Bello, Ozor Ignatius Ikemefuna, Mba Christian Ejuiwa, and Vivian Ugwu Abstract Background: The harmful effects of lead exposure, especially its effect on the nervous system, raise serious concerns worldwide. This study is aimed at evaluating the effect of Quercetin, Vitamin C and Pyridoxine on Haematology, biochemical and histology of the cerebral cortex on lead induced neurotoxicity using adult Wistar rat models. Materials and Methods: Forty male Wistar albino rats were divided into five groups: control, lead only, lead + Quercetin, lead + Pyridoxine, lead and Vitamin C. 100mg/kg/bw of lead was used to induce-toxicity for 7 days and treated with the following: Quercetin, Pyridoxine and Vitamin C were administrated orally respectively for 14 days. Hematological and biochemical samples were collected in three phases. The cerebral cortex was examined under light microscopy after H&E staining. Results: lead decreased all hematological parameters (PCV, WBC, RBC, HB and Platelets) examined and in the biochemical parameters, decreased slightly SOD, increased MDA and reduced significantly (p values) GSH. Quercetin, Vitamin C and Pyridoxine had strong curative effects on both hematological and biochemical parameters. On the histology, lead revealed mild glial cell infiltration. All treatment groups showed normal neuronal cells. Conclusion: Quercetin, Vitamin C and Pyridoxine showed neuroprotective effects against lead-induced neurotoxicity; therefore, they can be used as routine supplements against lead toxicity in endemic communities. Keywords: Quercetin, Pyridoxine, Lead, Ascorbic acid, neurotoxicity, lipid peroxidation Introduction Lead (Pb) is one of the most widely used heavy metals, especially in batteries, paint pigments, and plastics. This heavy use has produced local and global contamination of the air, soil, and water originating from lead-based pipes. The effect of exposure to Pb vary from mild to severe, depending on the degree of exposure, and is referred to as lead toxicity (Raymond, 2011). The nervous system (central and peripheral components) is the primary target of lead poisoning or toxicity (Brent, 2006; Bellinger, 2004). Pb toxicity is also known to induce a broad range of hematological, biochemical, and histomorphological dysfunctions in lab animals and humans (Hsu & Guo, 2002; Pande & Flora 2002). This is characterized by persistent vomiting, anemia, encephalopathy, lethargy, delirium, convulsions and coma, and death in extreme cases (Flora et al., 2006; Pearce, 2007). One of the possible mechanisms underlying Pb-induced toxicity or poisoning is its ability to induce oxidative stress in blood and other tissues, which contributes to the pathogenesis of poisoning by interfering with the delicate prooxidant/antioxidant balance that exists within the mammalian cells. Several investigators suggest a possible involvement of reactive oxygen species (ROS) in Pb-induced toxicity (Adonaylo & Oteiza, 1999; Pande & Flora, 2002; Hsu & Guo, 2002) where Pb increased lipid peroxidation indicator the malondialdehyde (MDA) and decreased the activities of antioxidants enzymes: glutathione peroxidase (GPx), and superoxide dismutase (SOD) in the rat brains (Kuhad & Chopra, 2007). Antioxidants have been identified to protect the neurons against various experimental neurode generative conditions (Kuhad & Chopra, 2007). Vitamin C, vitamins E and B6, zinc, and selenium are antioxidants used to reverse Pb-mediated toxicity by ameliorating oxidative stress status (Hsu & Guo, 2002). Flavonoids have now become a topic of interest due to their beneficial effects on different diseases. Quercetin is a natural flavonoid, ubiquitously found in common fruits and vegetables such as onions, broccoli, and apples (Sriraksa et al., 2016). Studies have reported that quercetin exhibits substantial antioxidants property, has the ability to scavenge free radicals, and aids many biological processes involved in oxidative stress (Formica et al., 1995). This study, therefore, evaluates the hematological, biochemical, and histomorphological effects of Quercetin, Vitamin C and Pyridoxine on lead-induced neurotoxicity in adult Wistar rats. Materials and Methods Procurement of Compounds, Chemical and Drugs 5g of Quercetin was procured from Zigma, Aldrich USA. Lead (Pb) weight of 5.2g was procured from registered chemical store at Ogbete main market Enugu Metropolis, Nigeria. 5g of Vitamin C (ascorbic acid) and Pyridoxine (Vitamin B6) were procured from Omaryon group of companies Enugu Metropolis, Nigeria. Animal Handling Forty (40) Wistar rats weighing between 150 – 250g were purchased from the animal house of the University of Nigeria, Nsukka, Enugu State, Nigeria. The rats were housed at the animal facility of the College of Medicine, Enugu State University of Science and Technology Enugu Nigeria. The rats were housed for a period of 4 weeks to attain desired weights and to get them acclimatized in their new environment. The rats were fed pelleted rat chow (Vital, Nig. Ltd.) and allowed water ad libitum. Thereafter, the rats were grouped into five (5) groups; each group had eight rats housed in two different cages 2 (n=4) per group. The rats were kept in ventilated cages at optimum temperature 28oC with 12 hours light/dark cycle, and humidity of 60%. The study was reviewed and approved by the Faculty Ethics and Research Committee. Induction of Lead Toxicity The used dose for lead induction was adopted from Highab et al., (2018), 100 mg /kg/bw of lead was administered orally for 7days across groups. Thereafter, Quercetin was administered as modified using methods of Tian et al., 2019, at 50mg /kg / bw). Vitamin C and Pyridoxine (Vitamin B6) were used as standard drug for the experiment, the doses were adopted from Razmkon et al., (2011) at dosage 500 mg /kg /bw for Vitamin C and 100 mg/ kg/ bwt for Pyridoxine (Vitamin B6).all treatment were administered via oral gavage for 14 days. Experimental Design
Hematological and Biochemical Evaluations Blood samples were collected from all the rats thrice to form a baseline, after induction and after treatment as well as parameter for post induction. Materials used for collection of samples are; capillary tubes and plain EDTA bottles. The following were evaluated; PCV, WBC, RBC, HB, and Platelets for hematological studies using standard protocols. While serum Superoxide dismutase (SOD), Malondialdehyde (MDA) & Glutathione (GSH) antioxidant marker enzyme were determined using the respective kit in accordance with manufacturers recommended protocols (Fortress diagnostic limited UK). Measurement of Malondialdehyde (MDA), a prototype of the thiobarbituric reactive substances (TBARS) as a biomarker of lipid peroxidation and oxidative stress using modified thiobarbituric acid method (Todorova et al., 2005).The IBM SPSS package (IBM Corp., IBM SPSS Statistics for Windows, Version 25.0, Armonk, NY, USA),was used to analyze the data. Descriptive statistics was evaluated for hematological and antioxidative markers and presented as means and standard deviations. An analysis of variance test (One-way ANOVA) was used for comparison of the means for the hematological, and antioxidant enzymes. The P values for comparing means were considered significant at p ≥ 0.05. Termination of Study and Tissue Collection On the 15th day (day one post treatment), the rats in all groups were sacrificed under light etherand the brains were harvested. The brains were fixed in 10% neutral formal saline for 48hrs. Thereafter the cerebrum were isolated for routine paraffin processing and stained with Haematoxylin and Eosin. Representative photomicrographs were captured after the interpretation. Results Hematological Analysis
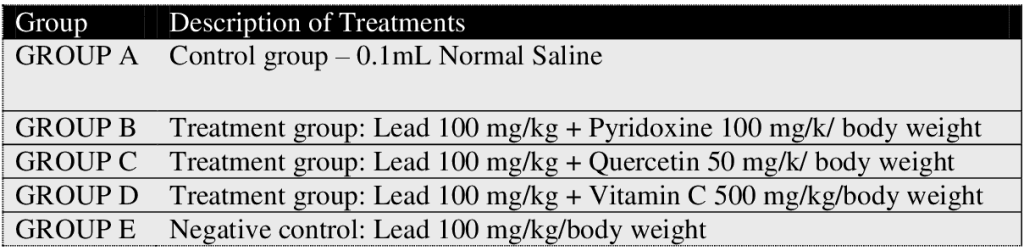
Figure 1: Shows PCV test results across groups. The PCV values for the baseline study, the post induction study (2nd study) and post treatment study (3rd study) were distinguished. In group E (Lead only), the PCV values regressed with continuous administration. Treatment groups B, C and D was observed to have increased PCV values after treatment which is similar when compared to observations in control group A. However, PCV values were not significant (P > 0.05) in paired sample correlations across groups in 2nd and 3rd study.
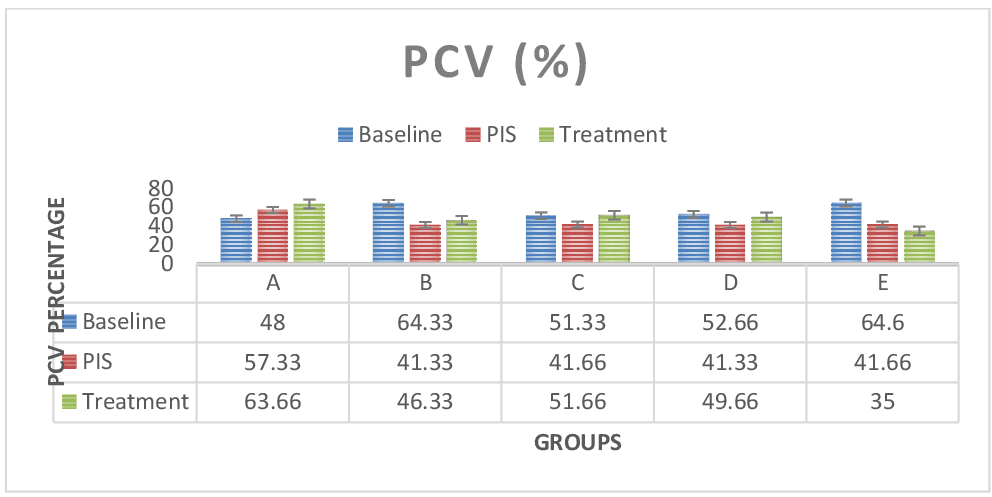
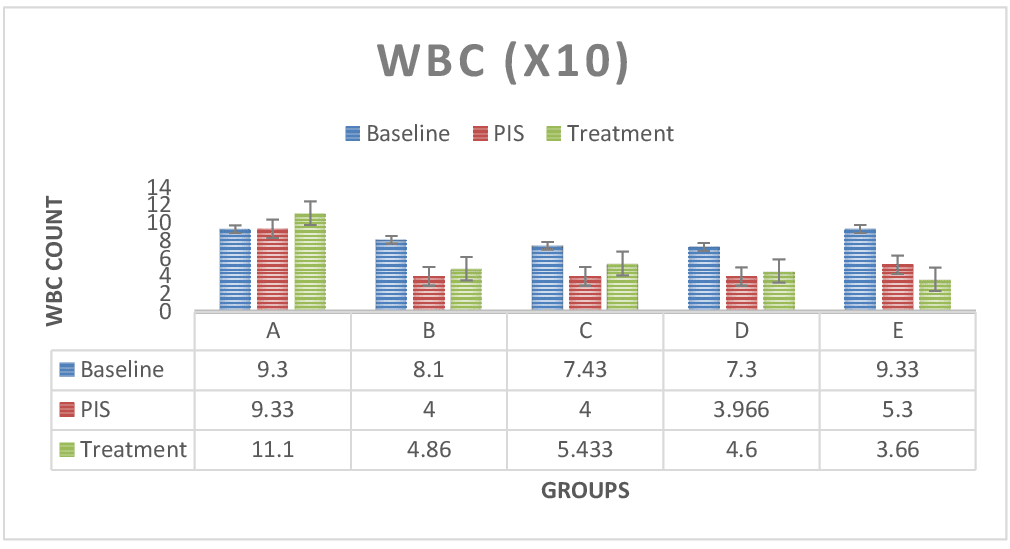
Figure 2: Shows WBC test results across groups. The WBC values for the baseline study, the post induction study (2nd study) and post treatment study (3rd study) were distinguished. In group E (Lead only), the WBC count was significantly lower (P < 0.05) compared to the control group A and treatment groups B, C and D. WBC values was observed to increase after treatment across treatment groups. WBC count was also significant (P < 0.05) in paired sample correlations across groups in 2nd and 3rd studies respectively. Figure 3: RBC test results across groups. The RBC values for the baseline study, the post induction study (2nd study) and post treatment study (3rd study) were distinguished. RBC values was reduced in the post induction study (2nd study) and increased at the treatment study across treatment groups (B, C & D) in comparison to lead only (E) group, which registered steady decline in RBC values. Paired sample correlates across groups in 2nd study and 3rd study groups also showed no significant difference (P > 0.05).
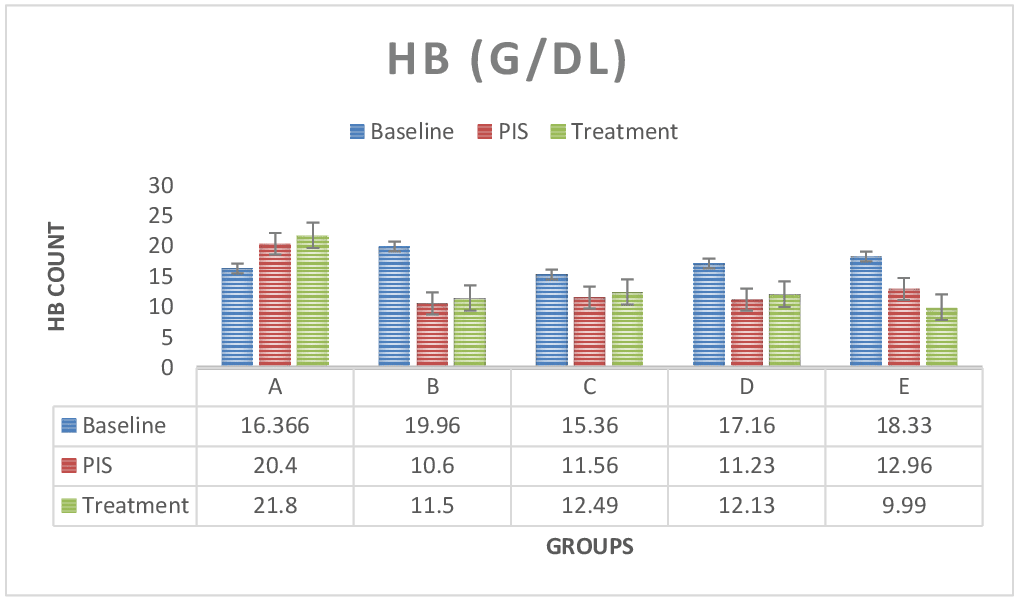
Figure 4: The figure above shows HB test results across groups. The HB values for the baseline study, the post induction study (2nd study) and post treatment study (3rd study) were distinguished. Hemoglobin values was lower with continuous administration in group E (lead only group), in comparison to control group A and treatment group B, C and D. however, HB values was seen to reduce in the post induction study results and increase in the treatment study result across treatment groups. hemoglobin was significant (P < 0.05) in paired sample correlation across groups in post induction (2nd study) and post treatment (3rd study) groups.
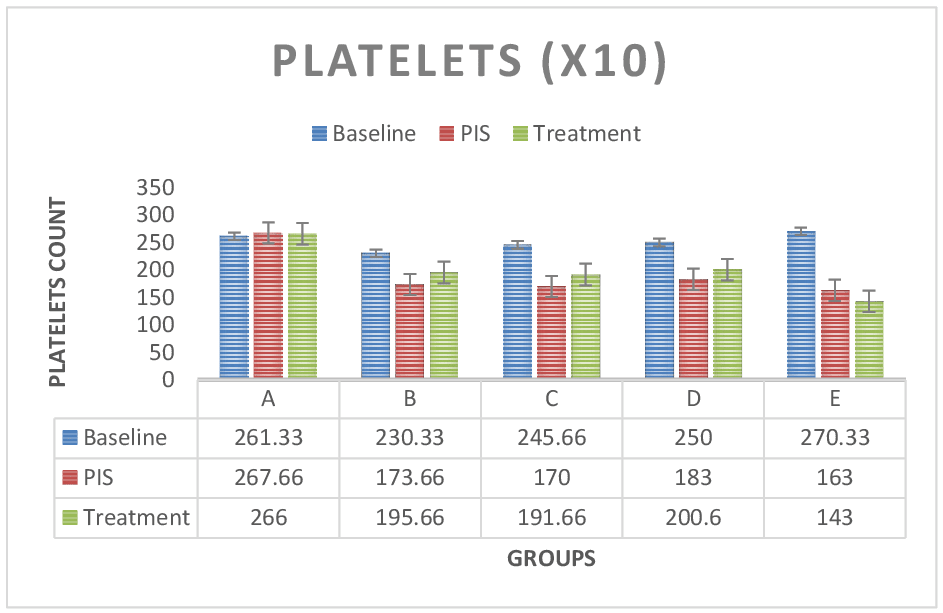
Figure 5: Platelets test results across groups. The platelets values for the baseline study, the post induction study (2nd study) and post treatment study (3rd study) were distinguished. Platelets values were continuously decreased in group E (Lead only) but there was no difference between treatment groups C, D and B. Platelet values were reduced in post induction study and increased in treatment group across treatment groups in comparison to control group A. However, paired sample correlations were significant (P < 0.05) across groups. Biochemical: Antioxidant Evaluation
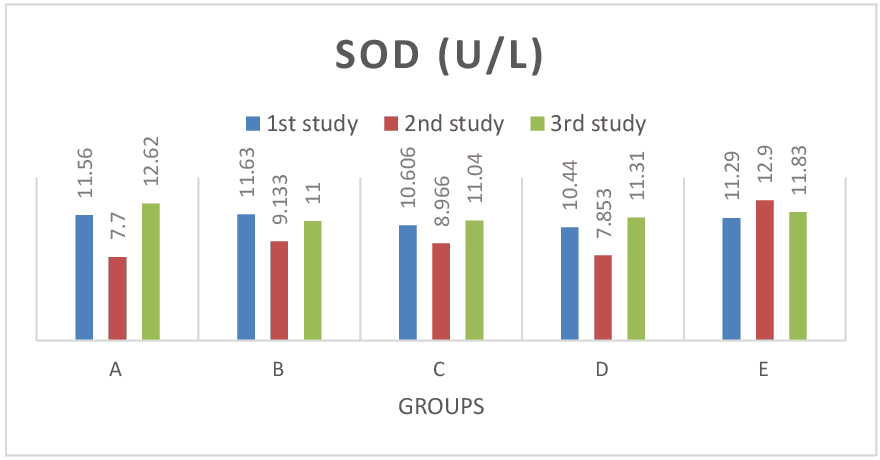
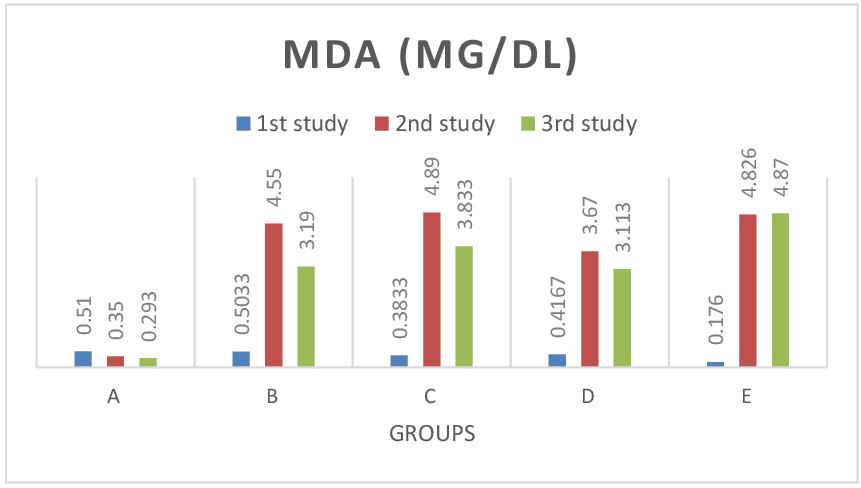
Figure 6: Shows SOD test results across groups. The SOD values for the baseline study, the post induction study (2nd study) and post treatment study (3rd study) were distinguished. There was no significant difference (P> 0.05) in SOD values across all groups. Also consistent with paired sample correlation of 2nd and 3rd study. Figure 7: Shows MDA test results across groups. The MDA values for the baseline study, the post induction study (2nd study) and post treatment study (3rd study) were distinguished. In group E (lead only), MDA values increased at continuous induction of lead in comparison to control group A, treatment group B (lead and pyridoxine), C (lead and quercetin) and D (lead and ascorbic acid) where values were reduced after treatment. Values were significant at P< 0.05 across groups. Also consistent with findings from paired sample correlation.
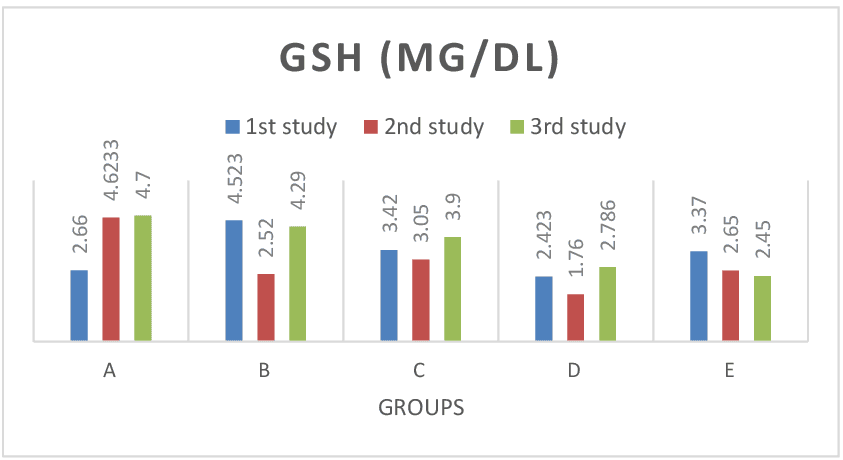
Figure 8: Shows GSH test results across groups. The GSH values for the baseline study, the post induction study (2nd study) and post treatment study (3rd study) were distinguished. GSH values continuously decreased in lead only group (group E), while there was notable increase in GSH values of treatment groups. There was significant difference (P> 0.05) in GSH post treatment values when compared with results of the baseline studies across group. Histomorphological analysis
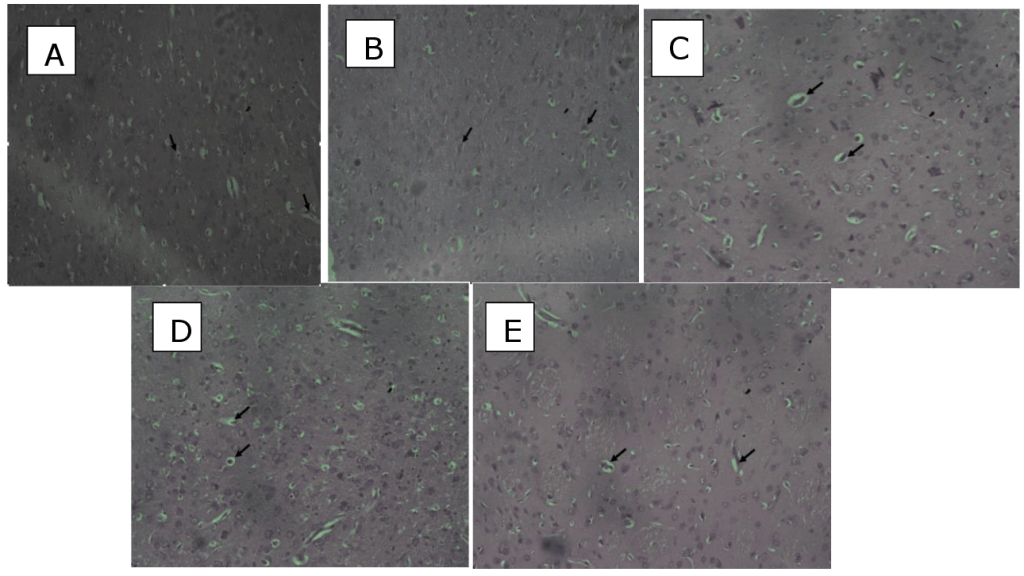
Figure 9: Sections of temporal cortices:(A)control rat (normal saline only)neuronal cells (arrow). cytoarchitecture appears normal. (B). Lead and Pyridoxine treated the cytoarchitecture appears normal with neuronal cells (arrow) ( C) Lead and Vitamin C the cytoarchitecture appears normal with neuronal cells (arrow) (D) Lead and quercetin treated the cytoarchitecture appears normal with neuronal cells (arrow) (E ) Lead only distortion of the cytoarchitecture (arrow) with mild glial cell infiltration. H&E. x200. Discussion Lead poisoning is known to cause iron deficiency which in turn is a strong contributing factor in the etiology of anemia (Staudinger & Roth, 1998). Anemia is classified as macrocytic, normocytic, or microcytic based on the size of red blood cells and the amount of hemoglobin (Sarma, 1990). A low level of hemoglobin reflects reduced PCV value, which is defined as the ratio of the RBC volume to the total blood volume (Beutler & Waalen, 2003). Additionally, anemia can be hypochromic when the MCHC value is low.In this study, lead toxicity was evident owing to the slightly decreased PCV and RBC values (Fig 1 & 3) across treatment groups. This is consistent with the findings of Mahdi et al., (2013) in a clinical study on lead-exposed workers. Also, WBC, HB, and Platelets count greatly decreased after induction of lead across all treatment groups (Fig 2, 4 & 5).This is consistent with the findings of previous studies (Omotayo et al.,2022; Mahdi et al., 2013; Nabil et al., 2012; Mugahi et al.,2003). Vitamin C was also observed to show curative effects by increasing PCV, RBC, WBC, HB and Platelets count after observed reduction by lead administration.This was attributed to its functional ability to donate reducing equivalents to prevent the formation of reactive oxygen that damage the RBC. This finding is in line with Ozor, (2020). In another study, ascorbic acid demonstrated a substantial rise in RBC, and WBC in ascorbic acid therapy (Masar et al., 2021). Pyridoxine also exhibited a similar effect as vitamin C on the PCV, RBC, WBC, HB, and Platelets counts. This is in line with the findings of Boleslaw et al., (2019). Quercetin enhanced all the blood parameters evaluated: PCV, RBC, WBC, HB, and platelets count. This is suggestive of haematopoietic enhancing property and affirms reports supporting quercetin supplementation enhanced RBC, WBC HB, PCV and Platelets count (Yahaya et al., 2020; Kasmi et al., 2018; Keskin et al.,2016; Selvakumar et al., 2013; Mahmoud et al., 2013). Antioxidants play a vital role in mitigating the effect of oxidative stress on tissues and observations in this study showed SOD values after induction of lead were reduced but not significant across groups. Other studies have earlier reported similar outcomes (Jin et al., 2006; Ahmed et al., 2008). However, significant a increases in SOD activity has been reported in lead-exposed workers (Kasperczyk et al., 2009; Rendon-Ramirez et al., 2014). Lead toxicity level is evident as an increased level of MDA recorded in this study. Thus is consistent with several studies which found MDA levels to be significantly higher in lead-exposed groups(Tenchova et al., 1997; Ye et al., 1999, Yucebilgiç et al., 2003, Gurer-Orhan et al., 2004, Kasperczyk et al., 2013, Oktem et al. 2004, Patil et al., 2006; Garçon et al., 2007; Ergurhan-Ilhan et al., 2008; Khan et al., 2008, Mohammad et al., 2008, Grover et al., 2010, Permpongpaiboon et al., 2011; Singh et al,, 2013). Further findings from this study also showed a significant decrease in GSH values on exposure to lead and several studies also identified a reduction in GSH levels in lead-exposed group (Mohammad et al., 2008; Feksa et al, 2012; Kasperczyk et al, 2013). However, few studies identified increased GSH levels in lead exposed group (Gurer-Orhan et al., 2004; Conterato et al., 2013). Evident from our findings quercetin considerably reduced MDA levels and increased SOD and GSH levels, thereby reaffirming its already described antioxidant properties (Yagmurca et al.,2015) and confirming the protective properties of Quercetin via the regulation of antioxidant and lip peroxidation entities (Polat et al., 2006; Ikizler et al., (2017). Vitamin C reduced lead-induced oxidative stress by decreasing MDA levels while increasing SOD and GSH levels. Vitamin C has the tendency to ameliorate oxidative stress levels (Bailey et al., 2011; Ozor, 2021). Vollaard et al, (2005) also posited that Vitamin C supplementation decreased serum MDA in exercise-induced oxidative stress. Pyridoxine also improved antioxidant markers. Pyridoxine supplementation exerted antioxidant and lipid profiles oflead-induced neurotoxicity (Tas et al., 2017). Histological observations revealed normal cytoarchitecture of the temporal cortices of the treated groups but not lead which revealed mild glial infiltrations. This was attributed to the lead effect on the cortex.Lead acetate caused mild histomorphological alterations in the brain (Jarrar et al., 2012) and dose- dependant cellular degenerative changes (Highab et al., 2018). Thus, lead exhibited a neurotoxic effect while the treatment quercetin, vitamin C, and pyridoxine exerted neuroprotective potential. This result was consistent with previous studies which reported the protective and antioxidant roles of quercetin, vitamin C, and pyridoxine (Vollard et al, 2005; Ikizler et al, 2017; Amanda et al, 2019; Ozor, 2021). Quercetin, particularly, enhances the survival of neuronal cells in the cerebral cortex (Khan et al, 2018). Conclusion Lead-induced oxidative stress consequently altered hematological parameters and temporal cerebral microstructure. The treatments with quercetin, ascorbic acid, and pyridoxine mitigated the adverse effect of lead via hematopoietic, antioxidant, and neuroprotective mechanisms. Conflict of Interest The authors declared that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest. Acknowledgement We thank the staff of the histology laboratory for providing the technical assistance. Author Contributions FBE: conception and design and supervised the research; MEC: wrote the draft; assembled and analyzed the histology; NIS and VU: performed the study, contributed to the discussion, reviewed; OII: review literature and edited the draft. All authors read and approved the manuscript. References Adonaylo, A., and P. Oteiza (1999). Lead intoxication: Antioxidant and oxidative damage in rat brain. Toxicology. 135: 77. Al-kurdy, M. (2021). Effect of ascorbic acid supplement on hematological parameters and some enzyme activities of Male Rabbits. Annals of the Romanian society for cell biology. 25.2758-2764. Amanda, D., Giustina, F, P. (2019). Vitamin B6 reduces oxidative stress in lungs and liver in experimental sepsis. Biological Sciences. An. Acad. Bras. Ciênc. 91 (04); https://doi.org/10.1590/0001-3765201920190434 Bailey, C D. M., Williams, J. A., Betts, D., Thompson, T. L. (2011). Oxidative stress, inflammation and recovery of muscle function after damaging exercise: effect of 6-week mixed antioxidant supplementation. European Journal of Applied Physiology, vol. 111, no. 6, pp. 925–936, Bellinger, D.C. (2004). Lead. Pediatrics.113:1016–1022. Beutler, E., Waalen, J. (2006). The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood.;107(5):1747–50. Brent, J.A. (2006). Review ofMedical Toxicology. Clin Toxicol.;44:355–355. Conterato, G.M., Bulcao, R.P., Sobieski, R., Moro, A.M., Charao, M.F., de Freitas, F.A.,et al. (2013). Blood thioredoxin reductase activity, oxidative stress and hematological parameters in painters and battery workers: relationship with lead and cadmium levels in blood. J Appl Toxicol 33(2):142–150. doi:10.1002/jat.1731 Ergurhan-Ilhan, I., Cadir, B., Koyuncu-Arslan, M., Arslan, C., Gultepe, F.M., Ozkan, G. (2008) Level of oxidative stress and damage in erythrocytes in apprentices indirectly exposed to lead. Pediatr Int 50:45-50 Feksa, L.R., Oliveira, E., Trombini, T., Luchese, M., Bisi, S., Linden, R.,et al. (2012). Pyruvate kinase activity and delta-aminolevulinic acid dehydratase activity as biomarkers of toxicity in workers exposed to lead. Arch Environ Contam Toxicol 63(3):453–460. doi:10.1007/ s00244-012-9786-z Flora, S.J., Flora, G, Saxena, G. Mishra,, M. (2007). Arsenic and lead induced free radical generation and their reversibility following chelation. Cell Mol Biol (Noisy-le-grand);53:26–47. Flora, S.J.S. (2002). Nutritional components modify metal absorption, toxic response and chelation therapy. J Nut Environ Med.;12:53–67. Formica, J.V., Regelson, W. (1995). Review of biology of Quercetin and Related Bioflavonoids. Food and Chemical Toxicology, 33, 1061-1080. http://dx.doi.org/10.1016/0278-6915(95)00077-1 Garçon, G., Leleu, B., Marez, T., Zerimech, F., Haguenoer, J.M., Furon, D., Shirali, P. (2007). Biomonitoring of the adverse effects induced by the chronic exposure to lead and cadmium on kidney function: Usefulness of alpha-glutathione S -transferase. Sci Total Environ 377:165–172 Ghanwat, G., Patil, A.J., Patil, J., Kshirsagar, M., Sontakke, A., Ayachit, R.K.. (2016). Effect of Vitamin C Supplementation on Blood Lead Level, Oxidative Stress and Antioxidant Status of Battery Manufacturing Workers of Western Maharashtra, India. J Clin Diagn Res. Apr;10(4):BC08-11. doi: 10.7860/JCDR/2016/15968.7528. Grover, P., Rekhadevi, P.V., Danadevi, K., Vuyyuri, S.B., Mahboob, M., Rahman, M.F. (2010). Genotoxicity evaluation in workers occupationally exposed to lead. Int J Hyg Environ Health 213:99–106 Gurer-Orhan, H., Sabir, H.U., Ozgunes, H. (2004). Correlation between clinical biomarkers of lead poisoning and oxidative stress parameters in controls and lead-exposed workers. Toxicology 195:147–154 Highab, S.M., Magaji, R.A., Muhammad, B.Y. (2018). Effects of lead poisoning and antidepressant drugs on the cerebral cortex of Wistar rats. Acta Scientific Pharmaceutical Sciences 2.5.16-21. Hsu, P., Guo Y (2002). Antioxidant nutrients and lead toxicity. Toxicology 180: 33–44 Ikizler, M., Erkasap, N., Dernek, S., Kural, T., Kaygisiz, Z. (2007). Dietary polyphenol quercetin protects rat hearts during reperfusion: enhanced antioxidant capacity with chronic treatment. Anadolu Kardiyol Derg; 7 (4): 404–410. Jarrar, B.M., Taib, N.T. (2012). Histological and histochemical alterations in the liver induced by lead chronic toxicity. Saudi J Biol Sci. Apr;19(2):203-10. doi: 10.1016/j.sjbs.2011.12.005. Jin, Y., Liaob, Y., Lua, C., Lia, G., Yue, F., Zhic, X., Xud, J., Liud, M., Yang, J., (2006). Health effects in children aged 3-6 years induced by environmental lead exposure. Ecotoxicol Environ Saf 63:313-317. Kalicki, B., Aneta, L., Krystyna, J., Monika, L., Justyna, M.J., Slawomir, L. (2019). Vitamin B6 improves blood parameters in rats fed a protein deficient diet and subjected to moderate, long term exercise. Cent Eur J Immunol. 44(1):23-32. Kasmi, S., Bkhairia, I., Harrabi, B., Mnif, H., Marrakchi, R., Ghozzi, H., et al.(2018). Modulatory effects of quercetin on liver histopathological, biochemical, hematological, oxidative stress and DNA alterations in rats exposed to graded doses of score 250. Toxicol Mech Methods ;28:12-22 Kasperczyk, S., Kasperczyk, J., Ostalowska, A., Zalejska-Fiolka, J., Wielkoszynski, T., Swietochowska, E., Birkner, E. (2009). The role of the antioxidant enzymes in erythrocytes in the development of arterial hypertension among humans exposed to lead. Biol Trace Elem Res 130:95–106 Kasperczyk, S., Slowinska-Lozynska, L., Kasperczyk, A., Wielkoszynski, T., Birkner, E. (2013). The effect of occupational lead exposure on lipid peroxidation, protein carbonylation, and plasma viscosity. Toxicol Ind Health. doi:10.1177/0748233713491804 Keskin, E., Dönmez, N., Kılıçarslan, G., Kandır, S. (2016). Beneficial effect of quercetin on some haematological parameters in streptozotocin-induced diabetic rats. Bull Environ Pharmacol Life Sci 2016;5:65-8. Khan, D.A., Qayyum, S., Saleem, S., Khan, F.A. (2008). Lead-induced oxidative stress adversely affects health of the occupational workers. Toxicol Ind Health 24:611–618 Kuhad, A., and Chopra, K. (2007). Curcumin attenuates diabetic encephalopathy in rat: Behavioral and biochemical evidence. European Journal of Pain 576: 34. Mahmoud, A.M. (2013). Hematological alterations in diabetic rats – Role of adipocytokines and effect of citrus flavonoids. EXCLI J;12:647-57. Mohammad, I.K., Mahdi, A.A., Raviraja, A., Najmul, I., Iqbal, A., Thuppil, V. (2008) Oxidative stress in.painters exposed to low lead levels. Arh Hig Rada Toksikol 59:161–169 Monteiro, H.P., Abdalla, D.S.P., Arcuri, A.S., Bechara, E.J.H. (1985) Oxygen toxicity related to exposure to lead. Clin Chen 31(10):1673–1676 Mugahi, M.N., Heidari, Z., Sagheb, H.M., Barbarestani, M. (2003). Effects of chronic lead acetate intoxication on blood indicies of male adult rat. DARU Journal of Pharmaceutical Sciences 11(4):147-151. Nabil, M.I., Esam, A.E., Hossam S.E., Yasmin, E.A.M. (2012). Effect of lead acetate toxicity on experimental male albino rat. Asian Pac J Trop Biomed Jan;2(1):41-46. Oktem, F., Arslan, M.K., Dündar, B., Delibas, N., Gültepe, M., Ergürhan-Ilhan, I. (2004). Renal effects and erythrocyte oxidative stress in long-term low-level lead-exposed adolescent workers in auto repair workshops. Arch Toxicol 78:681–687 Omotayo, B.1., Esther, F.A., Temitope, T.O., Bruno, C. (2022). Lead exposure – induced changes in hematology and biomarkers of hepatic injury: protective role of Trevo supplement. Environ Anal Health Toxicol; 37(2):e2022007. Ozor, I.I. (2021). Biomarker patterns and neuroprotective effects of ascorbic acid, progesterone and statin in induced traumatic brain injury using a rat model. Apr, Patil, A.J., Bhagwat, V.R., Patil, J.A., Dongree, N.N., Ambekar, J.G., Das, K.K. (2006). Biochemical aspects of lead exposure in silver jewelry workers in Western Maharashtra (India). J Basic Clin Physiol Pharmacol 17(4):213–229. Permpongpaiboon, T., Nagila, A., Pidetcha, P., Tuangmungsakulchai, K., Tantrarongroj, S., Porntadavity, S. (2011). Decreased paraoxonase I activity and increased oxidative stress in low lead – exposed workers. Hum Exp Toxicol 30(9):1196–1203. Polat, C., Tokyol, C., Kahraman, A., Sabuncuoglu, B., Yilmaz, S. (2006). The effects of desferrioxamine and quercetin on hepatic ischemia-reperfusion induced renal disturbance. Prostaglandins Leukot Essent Fatty Acids; 74 (6): 379–383. Raymond, F. (2011). Lead presentation on biology 464 aquatic toxicology Razmkon,, A., Sadidi, A., Sherafat-Kazemzadeh, E., Mehrafshan, A., Jamali, M., Malekpour, B., Saghafinia, M. (2011) Administration of vitamin C and E in sever head injury: a randomized double-blind controlled trial. Clin Neurosurg; 58:133-7. Doi: 10.1227/nue.0bo13e3182279a8f.PMID:21916138. Rendon-Ramirez, A.L., Maldonado-Vega, M., Quintanar-Escorza, M.A., Hernandez, G., Arevalo-Rivas, B.I., Zentella-Dehesa, A., Calderon-Salinas, J.V.M. (2014). Effect of vitamin E and C supplementation on oxidative damage and total antioxidant capacity in lead-exposed workers. Environ Toxicol Pharmacol 37(1):45–54. doi:10.1016/j.etap.2013.10.016 Roels, H.A., Buchet, J.P., Lauwerys, R.R., Sonnet, J. (1975). Comparison of in vivo effect of inorganic lead and cadmium on glutathione reductase system and δ-aminolevulinate dehydratase in human erythrocytes. Br J Ind Med 32:181–192 Sarma, P.R. (1990). Red cell indices. Clinical methods: The history, physical, and laboratory examinations. 3rd ed. Butterworths. Selvakumar, K., Bavithra, S., Suganya, S., Ahmad, B.F, Krishnamoorthy, G., Arunakaran, J. (2013). Effect of quercetin on haematobiochemical and histological changes in the liver of polychlorined biphenyls-induced adult male Wistar rats. J Biomark:960125. Sina, K., Mahdi, B.M., Seyed, R.M., Mohammad, T.S., Bita, D., Valiollah, M., Mahmoud, S. (2013). Clinical, Toxicological, biochemical and hematologic parameters in lead exposed workers of a car battery industry. Iran J Med Sci. Mar; 38(1):30-37. Singh, Z., Chadha, P., Sharma, S. (2013). Evaluation of oxidative stress and genotoxicity in battery manufacturing workers occupationally exposed to lead. Toxicol Int 20(1):95–100. doi:10.4103/0971-6580.111550 Sriraksa, N., Wattanathorn, J., Muchimapura, S., Tiamkao, S., Brown, K., Chaisiwamongkol, K. (2012). Cognitive-enhancing effect of quercetin in a rat model of Parkinson’s disease induced by 6-hydroxydopamine. Evid Based Complement Alternt. Med.:823206 Staudinger, K.C., Roth, V.S. (1998). Occupational lead poisoning. American family physician, 57, 719-726. Syeda, M., Zehra, B., Saiqa, T., Laraib, L., Sadia, S., Sidrah, S., Fizza, N., Sadia, S., Sarwat, Y., Amber, N., Saara, A., Irfan, S., Asia, A., Saida, H. (2021). Quercetin exhibits potent antioxidant activity, restores motor and non-motor deficits induced by rotenone toxicity; https://doi.org/10.1371/journal.pone.0258928 Taş, S., Sarandöl, E., Dirican, M. (2014). Vitamin B6 supplementation improves oxidative stress and enhances serum paraoxonase/arylesterase activities in streptozotocin-induced diabetic rats. ScientificWorldJournal.;2014:351598. doi: 10.1155/2014/351598. Tenchova, V., Petkova, V., Pavlova, S., Simeonov, I. (1997). Lipid peroxidation in chronic lead exposure. Probl Khig. 22:54–61 Todorova, I., Simeonova, G., Kyuchukova, D. (2005). Reference values of oxidative stress parameters (MDA, SOD, CAT) in dogs and cats. Comp Clin Path13, 190-194. Vollaard, N. B. J., Shearman, J. P., Cooper, C. E. (2005). “Exercise-induced oxidative stress: myths, realities and physiological relevance,” Sports Medicine, vol. 35, no. 12, pp. 1045–1062, Yagmurca, M., Yassar, Z., Bas, O. (2015). Effects of quercetin on kidney injury induced by doxorubicin. Bratisl med.j, 116(8). Doi: 10.4149/BLL-092. Yahya, P., Farhad, O., Mahin, N.Z., Mehrnaz, A., Azizollah, P., Mahboobe, H. (2020). Effects of Quercetin Supplementation on Hematological Parameters in Non-Alcoholic Fatty Liver Disease: a Randomized, Double-Blind, Placebo-Controlled Pilot Study. Clin Nutr Res.Jan;9(1):11-19 Ye, X., Fu, H., Zhu, J., Ni, W., Lu, Y., Kuang, X., Yang, S., Shu, B. (1999). A study on oxidative stress in lead-exposed workers. J Toxicol Environ Health A 56:161–172 Yucebilgiç, G., Bilgin, R., Tamer, L., Tukel, S. (2003). Effects of lead on Na + -K + ATPase and Ca +2 ATPase activities and lipid peroxidation in blood of workers. Int J Toxicol 22:95–97 |
||||||||
|