Journal of Medicine, Engineering, Environmental and Physical Sciences (JOMEEPS), Vol. 1, No. 1, June-July 2023. https://klamidas.com/jomeeps-v1n1-2023-02/ |
||||
|
Acid Hydrolysis of Cassava Peel Waste for Ethanol Production Ifeoma E. Madukasi Abstract Alternative substrates for ethanol production other than food crops will definitely lead to enhancement of farmer’s income and lead to food security. Cassava peel waste (CPW) rich in starch content was utilized in ethanol production. The cassava peel was collected from cassava tuber processors, milled (0.027mm) hydrolysed with 20% H2SO4 prior to fermentation with 5% baker’s yeast for 3 – 5 days. The fermented broth was distilled using distillation apparatus. Chemical properties of the distillate identified hydroxyl ion as its functional group while physical characterization of the distillate showed ethanol yield to be 33.74g/cm3. The odour compares favourably with absolute and industrial ethanol respectively. Keywords: cassava peel waste, ethanol production, acid hydrolysis, solid waste management
Globally, ethanol production has been mainly from food crops such as corn, sugarcane, potatoes and cassava tubers [1]. These are essential crops in Africa and Nigeria in particular as they are staple and economic food crops. However, the demand for ethanol production is on the increase in recent times due to its wide use in chemical and transportation industries and also because of its role in green house gas emission reduction [2]. Although Nigeria is one of the highest cassava producers and processors in the world, the abundant waste that accrues from cassava processing is yet to be adequately tapped. In Nigeria, cassava wastes are mainly left to rot away or burnt off to create space for the accumulation of new generation of waste heaps, emitting carbon dioxide and producing a strong offensive smell [3], [4]. Cassava peel waste (CPW) is high in cyanogenic glucosides and the pomace also high in biodegradable organic matters and may cause surface water pollution especially if they are stored under heavy rain or simply disposed of in surface waters [5]. This is typical scenario in Nigeria. The price of cassava has recently increased due to high demand of the tubers as it is now mandatory in Nigeria to include 10% to 20% high quality cassava flour (HQCF) into bread and confectionaries. This has seriously affected the availability of cassava tubers as numerous cassava food derivatives abound. It is also expected that the policy on 10% to 20% inclusion of HQCF into bread and confectionaries will spur more cassava growers consequently leading to more waste from cassava processing. Unattended wastes invariably will breed unhealthy environment which can hinder the sustainability of the production and processing process. Cassava processing produces large amounts of waste and is generally considered to contribute significantly to environmental pollution [6]. Thus, an attribute of global warming induced climate change. Generally, the long-term and broad-based impact of cassava processing on the environment can be corrected by proper cassava waste management [7], [6] which includes the use of cassava by-products as feedstuffs or as an alternative substrate for biotechnological processes which includes alcohol production thereby alleviating environmental issues [5]. Development of proper waste management for cassava waste is very important as it will solve many environmental challenges as well as alleviate poverty, and enhance the incomes of both urban and rural farmers. It will also enable the nation to meet the Millennium Development Goals (MDG) on environmental sustainability [8], [9]. Utilization of cassava peel waste for ethanol production is a carbon neutral process which will not affect the teeming populace that depends on cassava as food sources; rather, it will close the loop in cassava processing in Nigeria. Cassava flour has potentials for alcohol production due to its high content of fermentable sugar and stable shelf-life [10], [11] and also because of its complete and easier hydrolysis compared to other flours [9]. However, for its use in ethanol production, acids or cellulose enzymes catalyze the breakdown of cellulose into glucose, which are fermented to ethanol [12]. Enzyme hydrolysis for the production of ethanol from starchy materials is an expensive process [13]. Chemical hydrolysis gives advantages for short residence time than enzyme hydrolysis [14]. Basically, two different processes can be used to produce ethanol from starchy crops: dry milling and wet milling processes. In dry milling, the feed material is ground mechanically and cooked in water to gelatinize the starch. The enzymes or acids are then added to break down the starch to form glucose, which yeast ferments to ethanol. The separate hydrolysis and fermentation process uses distinct process steps for starch hydrolysis and glucose fermentation. The primary advantage of this process is that hydrolysis and sugar fermentation can be treated separately, thus minimizing the interaction between these steps [12]. Utilization of cassava flour in ethanol production will add to food insecurity, hence the proposed utilization of agro-waste rich in starch and cellulosic matter. The aim of this work is to produce bio-ethanol from cassava peel waste and other agro-waste materials that are high in starch and cellulosic matters through acid hydrolysis and fermentation processes.
2.1. Materials: The CPW was collected from FIIRO pilot garri processing plant, dried, milled with hammer milling machine (0.027mm).
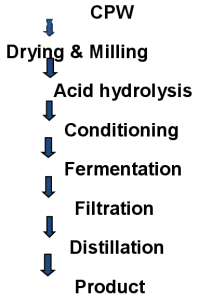
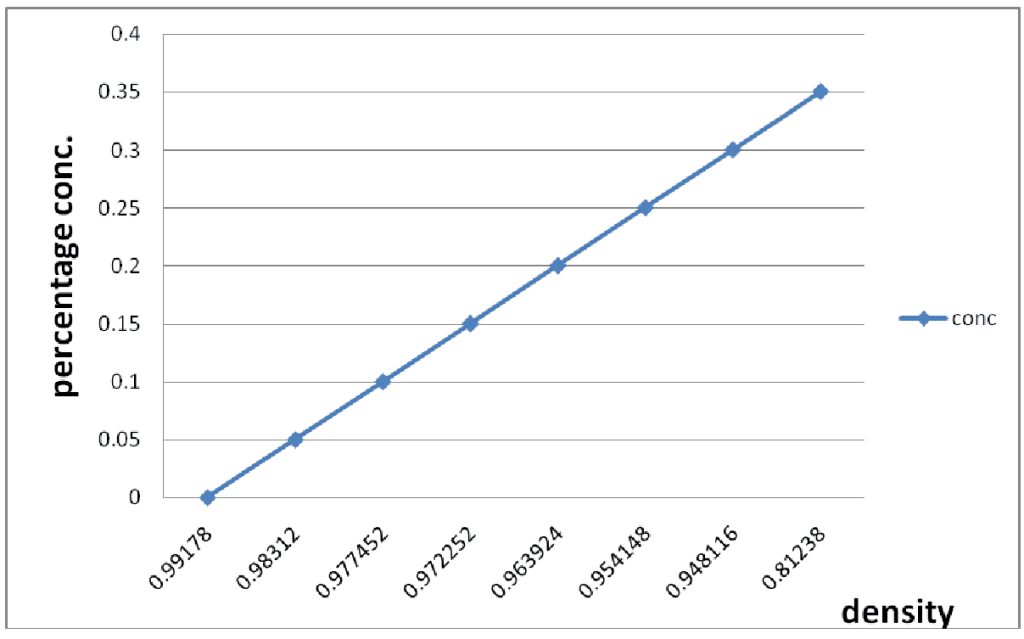
2.2. Pretreatment: Dry milling process was used before acid activation, this step enhanced visibility of the pores of the CPW for easier acid hydrolysis. In this method, the sieved CPW was boiled in water with constant stirring to a temperature of 70OC until the CPW gelatinized. 2.3. Acid Hydrolysis To the gelatinized sample, the prepared solution of the sulfuric acid was added with constant stirring until a homogenous mixture was formed. The solution was heated with constant stirring on a magnetic stirring hotplate until temperature of 65oC was reached. At this point, the texture and color of the solution changed. This was allowed to cool, filtered through No.1 whatman filter paper and the pH was adjusted to 4.5 with 0.1M Na0H. 2.4. Fermentation The CPW hydrolysate was fermented in an aspirator bottle (previously sterilized to exclude other microorganisms) with the baker’s yeast. The bottle was topped with straw to allow carbon dioxide to escape. Fermentation was done for 3 days and 5 days at room temperature. At the end of the fermentation period, the alcohol was separated from the extract using simple distillation. 2.5. Distillation process The distillation apparatus consisted of conical flask, condenser, splash head (to avoid the entrance of water vapors into the receiver) and the receiver. The fermented CPW hydrolysate was added into the conical flask and the set up was heated with a heating mantle at temperature between 75-80OC. The distillate collected was allowed to cool and the density was obtained. 2.6. Determination of Percentage Ethanol Concentration A slight modification of method according to [15] was used. Series of percentage (V/V) ethanol water solution were prepared and were weighed. The density of each of the prepared ethanol solution was calculated and a standard curve of density against percentage ethanol was plotted. The distillate was weighed and its density calculated. The percentage ethanol concentration of ethanol produced was obtained by comparing its density with the standard ethanol density curve. 2.7. Methodology: The sample was dried, milled with hammer milling machine (0.027mm), cooked in a pressure pot for 30 minutes prior to addition of 20% (18molar) sulphuric acid. The mixture was stood for 48hr and 5% baker’s yeast added into the content after pH adjustment to optimum condition of pH5.5. The mixture was agitated and 72hr to 96hr was allowed for complete fermentation. The liquor was filtered and the filtrate distilled using distillation apparatus. The process flow chart is as shown in Fig. 2.
Fig. 2: Process flowchart of ethanol production from cassava peel waste
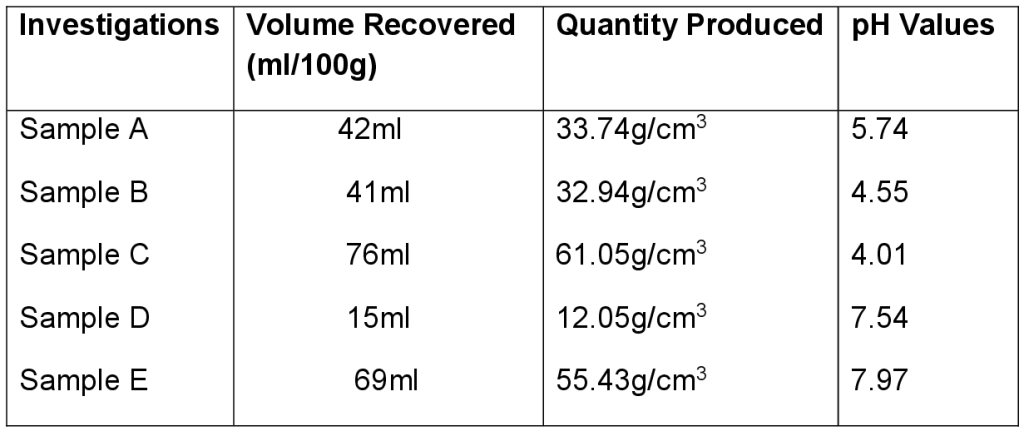
Table 1: Evaluation of Product Recovered From Preliminary Investigation
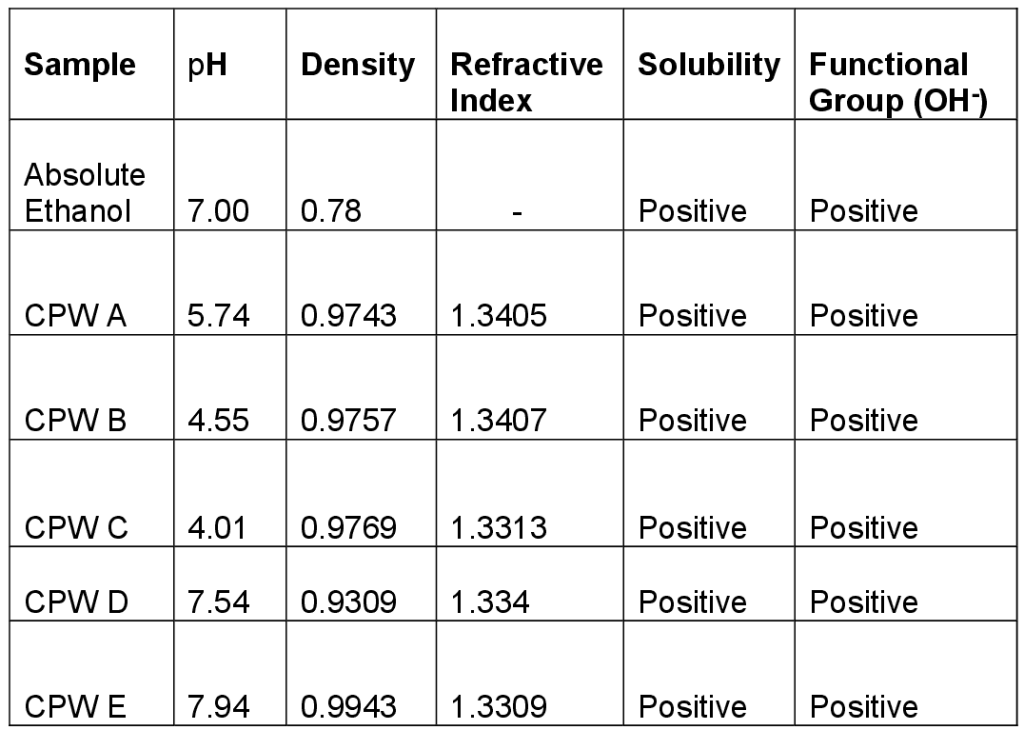
N/B: Density and pH of absolute ethanol= 0.8033g/cm3 and 7.00 Table 2: Determination of Physical & Chemical Properties of Ethanol Produced from CPW
Fig 3: Determination of Ethanol Concentration The distillate collected was measured with a measuring cylinder and expressed as quantity of ethanol produced in g/l by multiplying the volume yield of the distillate by ethanol density. From the trial investigations as shown in Table 1 above, CPW ethanol yield is comparable with literature value of 40% and above as recorded by [13]. The CPW yield was in the range of 33 – 60g/cm3 for the sterilized hydrolysate and 15-28g/cm3 for the non-sterilized hydrolysate. This is an indication that the baker’s yeast would have competed with other micro-organisms that hindered its activity otherwise depleted the available sugar in the case of non-sterilized hydrolysate. Table 2 shows the physical and chemical properties of ethanol as to ascertain the strength level and its degree of purity as compared to standard (absolute ethanol). In addition, CPW granules were extracted by crushing and pressure cooking to gelatinize the starchy content which was then treated with acid at 50 – 60 degree Celsius for 30 minutes to convert the starchy content into sugar (maltose). Addition of yeast in adequate proportion at room temperature converted the maltose to glucose with the aid of maltase while zymase also found in yeast decomposed glucose to ethanol with evolution of carbon dioxide. In summary, acid hydrolysis of carbohydrate to sugar is favoured in that it is faster with shorter residence time than enzyme hydrolysis.
In summary, acid hydrolysis of carbohydrate to sugar is favoured in that it is faster with shorter residence time in ethanol production. The use of cassava peel waste in ethanol production has shown that cassava has a holistic value and if properly harnessed will improve the economic values of the cassava growers in Nigeria. In addition, the work indicates that proper waste management is a tool to wealth creation and resource recovery. References: [1] Yan S; Yao J; Yao L; Zhi Z; Chen X and Wu J (2013). Fed batch enzymatic saccharification of food waste improves the sugar concentration in the hydrolysates and eventually the ethanol fermentation by Saccharomyces cerevisiae HO58. Braz. Arch. Boil. Technol. Vol. 55 No. 2 curitiba [2] Nuwamanya E; Chiwona-Karltun L; Robert S; Baguma KY (2012). Bio-Ethanol Production from Non-Food Parts of Cassava (Manihot esculenta Crantz). Ambio. 41: 262-170. [3] Aro S.O; Aletor V.A (2010). Proximate composition and amino acid profile of different fermented cassva tuber wastes collected from a cassava starch producing factory in Nigeria. http://www.lrrd.org/lrrd24/3/aro24040.htm [4] Adebayo, A. O (2008). Using cassava waste to raise goats. Project 2008-4345. World Bank Development Marketplace. http://wbi.worldbank.org/development market place/idea/using-cassava-waste-raise-g… [5] Pandey A; Soccol C.R; Nigam P, Brand D, Mohan R, Roussoss S (2000c). Biotechnology potential of agro-industrial residues, Part II. Cassava bagasse. Biores. Technol. 74: 81-87. [6] FAO (2011). Food Waste Repository programme of the Food and Agricultural Organisation of the United Nations [7] Ademiluyi F.T and Mepba H.D (2013). Yield and Properties of Ethanol Biofuel Production from Different Whole Cassava Flours. Hindawi Publishing Corporation. ISRN Biotechnology. 2013: ArticleID918481,6pages (http://dx.doi.org/10.5402/2013/916481). [8] Rabah A.B, Oyeleke S.B, Manga S.B, Hassan L.G (2011). Microbial Pretreatment of Rice husk and groundnut shell for bioethanol Production. International Research Journal of Microbiolgy. 2:253-258 [9] Kim S; Dele E (2005). Global potential Bio-ethanol production from wasted crop and crop residue. Biomass Bioenergy, 26: 361-347. [10] Holt J.G; N.R. Kneg, P.H. Sneath, T.J. Stanley and S.T. Williams (1994). Bergy’s manual of determinative bacteriology, pp: 983-987. [11] Humprey C.N; U.C. Okafoagu (2007). Optimization of ethanol production from Garcinia kola (bitter kola) pulp Agro Waste. African Journal of Biotechnology, 6(17):2033-2037. [12] Agulejika E.O; Olabode F.I; Babatunde K.A (2005). Ethanol production from waste fruits. International Journal of Food and Agricultural Research. Vol. 2 No 2: 190-194. [13] Muratianto BW; Chandra BA (2008). Production of bio-ethanol from cassava peel waste (CPW) using sweet corn enzyme powder. Semesta Bilingual Boarding School Semarang Indonesia [14] Echegi USC; Ejikeme PCN; Ejikeme EM (2013). Effects of process factors on the synthesis of bioethanol from cassava tubers using H2SO4 as catalyst. International Journal of Engineering and Science (IJES) Vol. 2, Issue 5, 1-9. [15] Oyeleke, S.B; Jibrin N.M (2009). Production of bioethanol from guinea cornhusk and millet husk. African Journal of Microbiology Research Vol. 3(4) pp. 147-152 |
||||
|