Global Online Journal of Academic Research (GOJAR), Vol. 2, No. 2, March-April 2023. https://klamidas.com/gojar-v2n2-2023-01/ |
||||
|
Use of Fenton’s Reagent for Pollutants Removal in Pharmaceutical Effluent By E.I. Madukasi and O.B.Tojuola
Abstract Effluent from a pharmaceutical factory producing a single chemical product was treated on a bench scale with advance oxidation process (H2O2 & Fe2+). The effluent was as a result of the factory treatment of the pharmaceutical wastewater by UASB (up flow anaerobic sludge bed) and a SBR (sequencing batch reactor) process. The chromatechemical oxygen demand (CODcr) range of the discharged effluent was between 8000-10,000mg/L with some residual recalcitrant compounds. The residual recalcitrant compounds which were measured by gas chromatography mass spectrometry (GC-MS) mainly consisted of alcohols, phenols and nitrogenous and sulfur compounds. The experimental variables studied include dosages of Fe2+, H2O2 and mixing speed. The result showed that the oxidation by Fenton’s reagent was best when concentration of iron (II) sulfate and hydrogen peroxide were [Fe2+]=1.093mmol/L; [H2O2] = 2.5mmol/L at pH = 3.0 for 30min at 80rpm; followed by conditioning with lime (1%) to pH = 8.0 where coagulation by iron hydroxide took place over 20min. Under these optimal operating conditions, the maximum removal efficiency for CODcr, Color and the aromatic compounds were 56%, 95% and 90% respectively. Keywords: Fenton reagent, pharmaceutical effluent, hydrogen peroxide, ferrous salt, effluent treatment, chemical treatment
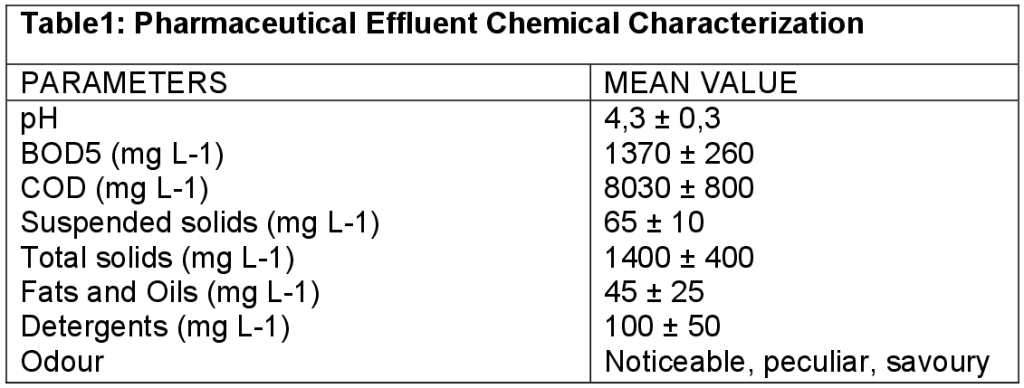
Introduction The process of toxicity reduction in pharmaceutical wastewater is of paramount importance due to known fact that substances synthesized in pharmaceutical industry are either toxic inhibitory compound or structurally complex organic chemicals that are resistant to biological degradation and consequent accumulation in the environment as well as possible carcinogenic and mutagenic effect (Jia et al, 2015; Ronak & Shweta, 2015; Balcioglu & Otker, 2003). This makes conventional treatment methods inadequate for the treatment of pharmaceutical wastewater. One of the possible methods for their degradation and removal is chemical oxidation, especially advanced oxidation processes (AOPs) using for example O3/H2O2 (peroxone); O3/H2O2/UV; O3/UV; H2O2/UV; Tio2/UV and Fenton reactions. These processes involve the in-situ formation of highly reactive hydroxyl radicals (•OH), which react quickly and non-selectively with almost all organic pollutants (Vrushali & Gawande, 2015; Tijani et al., 2016; Sanchis et al., 2013). One of the most important advance oxidation processes used to generate hydroxyl radicals employs the Fe2+/H2O2 system where the catalyst (ferrous ions) is dissolved in water, thus being known as Fenton process (Yuan et al., 2013). Fenton’s reaction is one of the most effective methods of oxidation of organic pollutants that are oxidatively degraded by hydroxyl radicals generated from H2O2 in the presence of Fe3+ as a catalyst (Hartmann et al., 2010; Maezono et al., 2011). Fe2+ + H2O2 ————> fe3+ + OH + OH• equation1. When ferrous salts are used, the hydroxyl radical is produced immediately by the rapid reaction between ferrous ion and H2O2 (equqtion1). Fe3+ can also be used to decompose H2O2 and to produce oxidative radicals in the Fenton-like process. With Ferric salts, the hydroxyl radical is produced in a two-stage process with the slow reaction between ferric ion and H2O2 (equation2) followed by the rapid reaction between the produced ferrous ion and additional hydrogen peroxide [Kiwi et al.,1993]. Fe3+ + H2O2————> fe2+ +HO2• + H+ equation 2. In most applications, it does not matter whether fe2+ or fe3+ ions are used to catalyze the reaction, although some authors (Pera-Titus et al., 2004; Walling & Amarnath, 1982) suggested that if low doses of Fenton’s reagent are used ferrous ions may be preferable. The efficiency of Fenton’s process depends on H2O2 and fe2+ concentrations and the pH of the reaction. According to some previous researcher’s report, pH value should range from 3 to 5 (Mohammadine et al., 2014; Navalon et al., 2010 and Maezono et al., 2011). Fenton reagent was found to be very effective in treating various industrial wastewater components, including aromatic & aliphatic compounds (Barbusinski & Filipek, 2001), a wide variety of dyes (Hsueh et al., 2005) as well as many other substances, including pesticides (Barbusïñki & Filpek., 2001). In this work, we present the oxidative treatment of pharmaceutical effluent that contained some recalcitrant compounds by Fenton reaction, using COD as determinand for the parameter only. This is because the pharmaceutical effluent will be subjected to critical biological treatment but effort was geared towards understanding the behavior of the effluent in a chemical treatment method hence the use of a single and most suitable parameter for efficiency determination. Material & Methods Wastewater: The pharmaceutical effluent is from a medium scale drug manufacturing plant situated in Ota near Lagos State Nigeria. The presence of toxic compounds in wastewater was both due to the factory product as well as chemicals used in sterilizing the manufacturing equipment. The intermittent cleaning and disinfecting of the tanks used in the production as well as domestic utilization of the tap water make up the wastewater coming out of the plant. The plant manufacturing line is operated as batch reactor. All the characteristics of the wastewater (Table1) were measured according to procedures described in standard methods (AWWA, 1995)
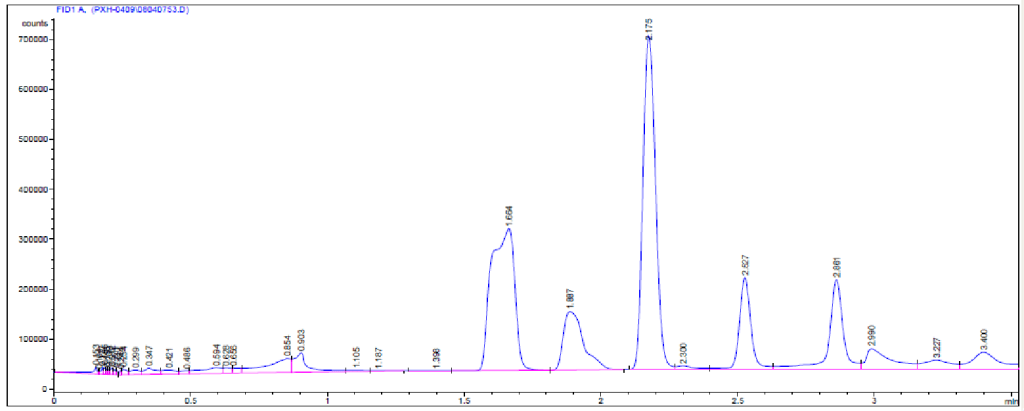
Experimental procedure: The following parameters of Fenton’s reaction were examined and optimized; hydrogen peroxide and ferrous concentration, [fe2+]: [H2O2] ratio, and initial pH of the reaction. A liter of wastewater was added into one liter Erlenmeyer flask which served as the reactor, acidified with H2SO4 (Fenton reaction is effective at acidic pH range). Since the initial pH of the wastewater is above 5.0, the sample was acidified to the desired value in the pH range of 2.5 –4.0. After which various doses of 30% H2O2 and solid FeSO4.7H2O were added, the mixture was vigorously stirred for 1hour at 80rpm (oxidation process), then pH was adjusted to 8.0 with 1% lime and coagulated at 30rpm for 20min. it was allowed to sediment and chemical oxygen demand (COD) and residual H2O2 were determined in the clear solution. COD tests were made after total removal of residual H2O2. The residual H2O2 can increase the COD value since it acts as a reductant, especially in the chromate based analysis of COD determination. Talinli and Anderson (1992) investigated the reducing effect of H2O2 on K2Cr2O7 and they showed linear relationship between concentration of H2O2 and COD. Residual H2O2 removal This was achieved by raising the pH of the solution (pH=10.5) at high temperature (45) in the presence of fe3+ with stirring at 65rpm and was sit overnight (Walling & Amarnath, 1982; Krzysztof Barbusinski, 2009). Analytical methods Aromatic and aliphatic compounds were analyzed by Infrared spectrometer (FTIR spectrometer) and confirmed with gas chromatography – mass spectrometry (GC– MS) as shown in fig1. The trace element analyses were carried out with inductive coupled plasma (optimal emission spectrometer, optima 5300DV). COD (closed reflux, trimetric method). Concentration of residual H2O2 was analyzed by the KMnO4 method.
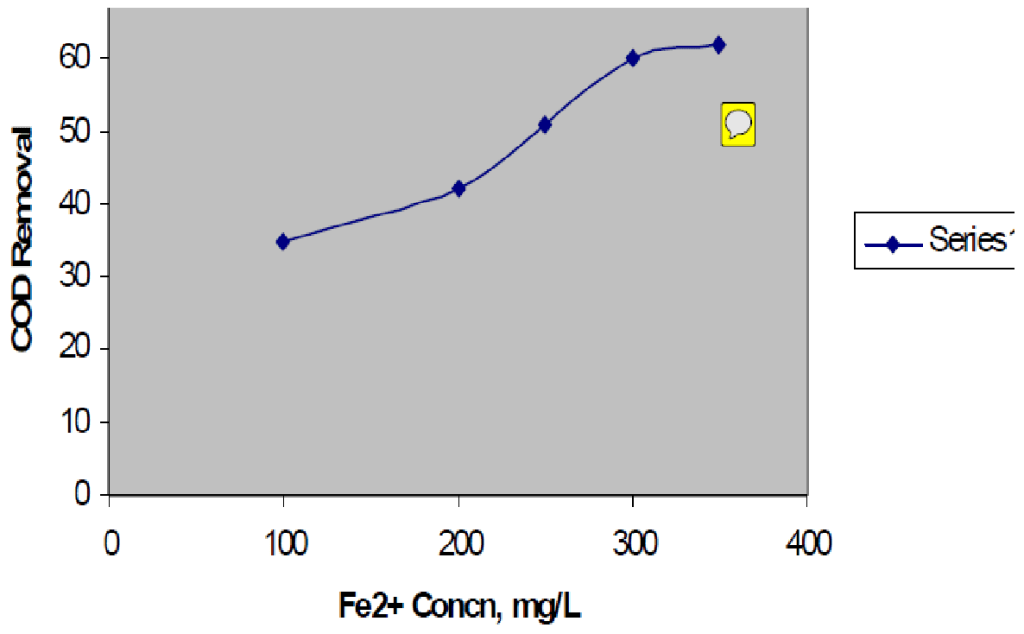
Statistical Analysis Data are presented as mean ± SD of triplicate determinations and descriptive statistics was conducted using Microsoft Excel (2016). Result and Discussion Effect of Fe2+ concentration It was shown that as the concentration of Fe2+ increased, the COD removal increased until a point where further addition of iron becomes inefficient. The feature of an optimal dose range for the iron catalyst is characteristics of Fenton reagent although the range varies for different wastewater. Typical ranges are [Fe2+]:[H2O2 ] = 5– 25 (Pere-Titus et al., 2004). Figure2 shows the effect of iron dosage on COD removal using different initial H2O2 concentrations. The result showed that the efficiency of Fenton reaction depends on the concentration of fe2+ as occurs in the classical Fenton reagent process. (Rajesh & Raman, 2013) have demonstrated that the COD removal is nearly the same using either fe2+ or fe3+ in the degradation of a textile wastewater by the Fenton process. The maximum COD removal achieved in this study was between 40 – 56% depending on the hydrogen peroxide dosage.
FIG. 2: Effect of Fe2+ Concentration variation. H2O2 @ 1.6M; pH = 2.5; Mixing speed = 100rpm @ 30min Effect of temperature and pH An initial experiment was carried out at room temperature and at 50oC, to show the effect of temperature on COD removal efficiency but no significant differences were observed in the treatment efficiency for the tested temperatures (data not shown). Thus all further work was carried out at room temperature. Research findings indicate that the temperature of the wastewater almost does not affect the efficiency of COD removal in Fenton’s oxidation (Peral et al., 2002; Casero et al., 1997), although the redox reaction can be accelerated by rising the temperature as expected. The time required for the oxidation to be completed at room temperature was about 15–20 times longer than at 50oC which required several minutes (data not shown). When tested with initial pH range of 2.5 – 4.5, no significant differences in the treatment efficiency were observed although pH 3.5 showed slightly better results. This finding is in adherence with the recent research reports that suggest that the optimum pH for Fenton oxidation is between 3–5 and that it is independent of the nature of the wastewater. (Tambosi et al., 2006; Hsueh et al., 2005) all observed that pH affects significantly the degradation of organics by Fenton reaction and acidic condition is required to produce sufficient hydroxyl radicals by the decomposition of hydrogen peroxide catalyzed by ferrous ions. In a recent study, (Zhang et al., 2005) reported the optimum pH as 2.5 for the treatment of landfill leachate by Fenton’s oxidation.
Effect of H2O2 concentration The degradation rate of organics in wastewater increases as the concentration of H2O2 increases until a critical H2O2 concentration is achieved (Huseh, et al., 2005). Above this critical concentration, the degradation rate of organic compounds decreases as a result of the scavenging effect, according to equation3 H2O2 + OH• ————> HO2 • + H2O (3) Fig.3 clearly shows that as the concentration of H2O2 increases, there was an increase in the COD removal efficiency up to the optimum concentration of 15ml/L. Above this amount of H2O2, there was decrease in the efficiency removal.
Conclusion The Fenton process could be applied to pharmaceutical effluent. The COD removal efficiency using oxidation was greatly affected by the initial pH of the solution. The most efficient reaction was observed at a pH of 3.0 and the optimum coagulation pH range to maximize the COD removal efficiency was between 6.0 – 8.0. For a pharmaceutical wastewater with a COD range of 8000 – 10000mg/L, average COD removal efficiency was highest when the ratio of H2O2/Fe2+ was about 150–250. At 0.3M H2O2 and 0.012M the optimum COD removal efficiency of 65% was achieved. Fenton reagent could be used to treat pharmaceutical wastewater that contains some constituents that are extremely toxic to biological processes hence viewed as biocides. Fenton’s reaction proves to be an efficient treatment technology when biological treatment is not feasible. Acknowledgements The authors wish to acknowledge the technical support given by FIIRO and COOU respectively.
REFERENCES Alpha Standard Methods for the Examination of Water and Wastewater, 18th edn. American public Health Association Washington, DC, 1992. Balcioglu, A. and Otker,M.(2003). Treatment of pharmaceutical wastewater containing antibiotics by O3 and O3/H2O2 processes, Chemosphere 50: 85–95. Barbusïñki, K. and Filpek, K. (2001). Use of Fenton’s Reagent for Removal of pesticides from Industrial Wastewater. Polish Journal of Environmental Studies. 10(4): 207 – 212. Barbusinski, K. (2009). Fenton Reaction-Controversy concerning the chemistry, Journal of society of ecological chemistry and engineering, 16(3):348-358. Casero, D., Sicilia, S. R, and Perez, D. B. (1997). Chemical degradation of aromatic amines by fenton’s reagent, Water Res.31: 1985 – 1995. Hsueh,C. L; Huang, Y.H., Wang, C.C. and Chen, C.Y. (2005). Degradation of azo dyes using low iron concentration of Fenton and Fenton-like system, Chemosphere, 58: 1409-1414. Huicen, Z., Weimin, G., Zhemin, S., Qingli,T., Wenchao, J. and Lijuan, J. (2015). QSAR models for degradation of organic pollutants in ozonation process under acidic condition. Chemosphere 119:.65-71. Jimoh. O., Tijani, O., Fatoba, O., Omotola, O., Babajide, L. and Petrik, F.(2016). Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants.A review. Environmental chemistry letters. 14(1):27-49. Kiwi, J., Dulgarin, C., Peringer, P. and Gratzel, M. (1993). beneficial effects of homogenous photofenton pretreatment up on the biodegradation of anthraquinone sulfonate in wastewater treatment. Appl. Catal. B. Environmental. .3: 85-88. Maezono, T., Tokumura, M., Sekine M., and Kawase, Y. (2011). Hydroxyl radical concentration profile in photo-Fenton oxidation process: Generation and consumption of hydroxyl radicals during the discoloration of azo-dye Orange II. Chemosphere 82(10):1422-1430. Marin, S., Ramos, A. J., Cano-Sancho, G., and Sanchis, V. (2013). Mycotoxins: Occurrence, toicology and exposure assessment (Review). Food and Chemical Toxicology 60: 218–237. Mohammadine, E. H., Abdelmajid, Regti., Rachid, Laamari., Rachid, Mamouni. and Nabil, Saffaj. (2014). Use of Fenton reagent as advance oxidative process for removing textile dyes from aqueous solutions. Journal of Material and environmental science 5(3): 667-674. Navalon, S., Alvaro, M. and Garcia, H. (2010). Heterogeneous Fenton catalysts based on clays, silicas and zeolites. Applied Catalysis B: Environmental. 99(1-2):1-26. Pere-Titus, M., Garcia-Molina, V., Banos, M. A., GimeneZ, J., Esplugas, S. (2004). Degradation of chlorophenols by means of advanced oxidation process: a general review, Applied CatalysisB: Environmental, 47: 219-256. Pérez, M., Torrades, F., Doménech, X. and Peral, J. (2002). Fenton and photo-Fenton oxidation of textile effluents, Water Res. 36: 2703–2710. Rajesh, N., and Raman, S. (2013). Treatment of Pharmaceutical Sludge by Fenton Oxidation Process. International Journal of Chemical Engineering and Applications. 4(6): 359-364. Ronak, S., Shweta, V. (2015). Fenton’s Reagent for the Treatment of Pharmaceutical Industry Wastewater. International Journal of Science and Research. 4(7): 3093-3095. Talinli, I. Anderson, G. K. (1992). Interference of hydrogen peroxide on the standard COD test. Wat. Res. 26: 107- 110. Tambosi, J.L., Domenico, M., Schirmer, W.N., Jose, H.J., Moreira-Reaina, F.P.M (2006). Pre-oxidation and coagulation on pper &pulp wastewater by fenton-lik process. J. Chem. Tech and Biotech. 81(8): 1426-1432. Vrushali, P. and Sagar, G. (2015). .An overview of the Fenton Process for Industrial Wastewater. Journal of Mechanical and Civil Engineering. 21:127-136. Walling, C., Amarnath, K. (1982). Oxidation of mandelic acid by Fenton’s reagent. J. Am. Chem. Soc. 104: 1185-1189. Zhang, H., Choi, H. J. and Huang, C. P. (2005) Optimization of Fenton process for the treatment of landfill leachate, J. Hazard. Mater. 125: 66-174. |
||||
|